




Hello from Doctor Pack!
A leader and a trusted Packaging and Drug Delivery Device partner to leading global Healthcare, Pharmaceutical, Biotechnology, Diagnostics companies, Hospitals, Retail Pharmacies, and Medical Contract Packers
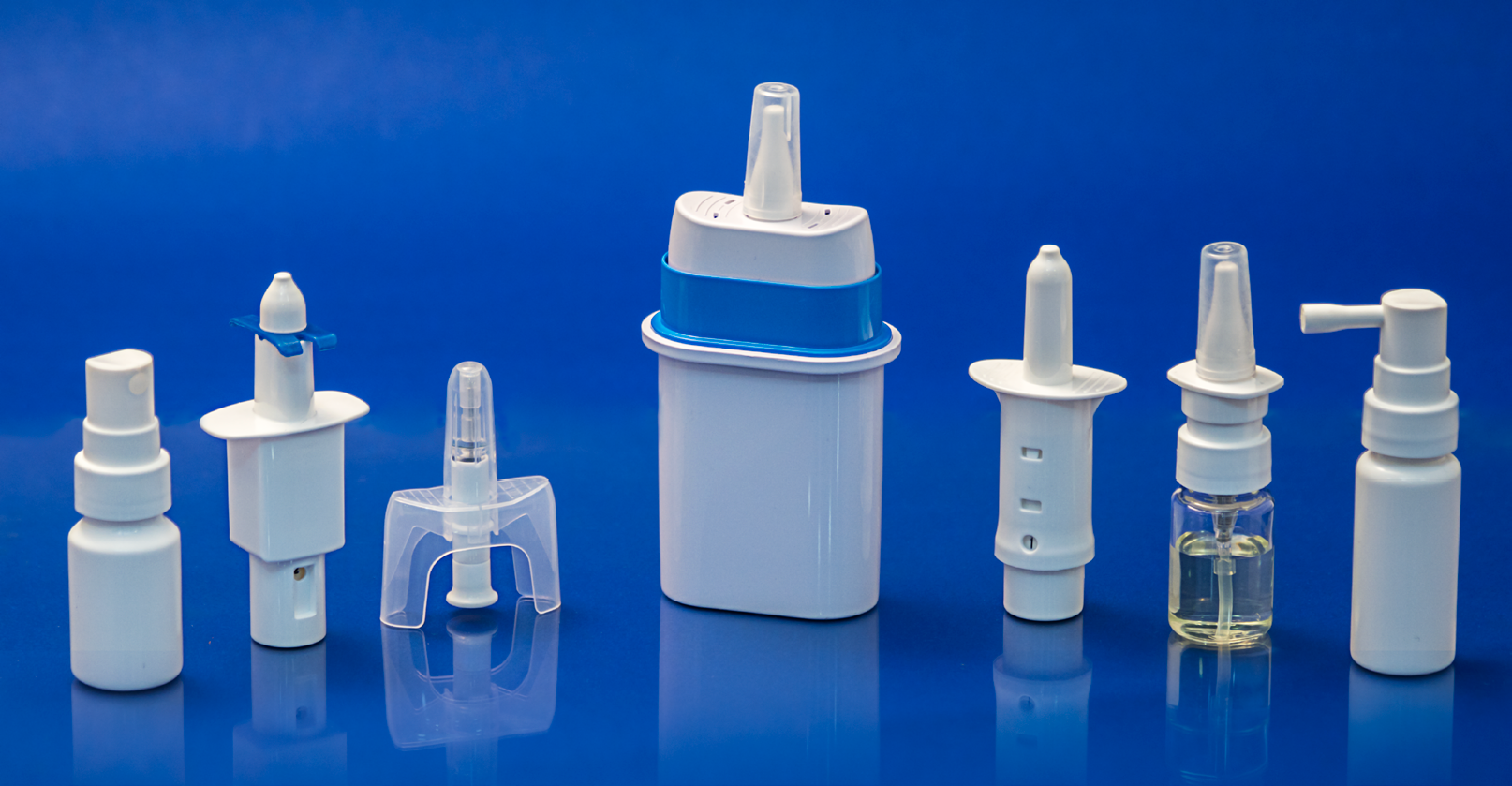
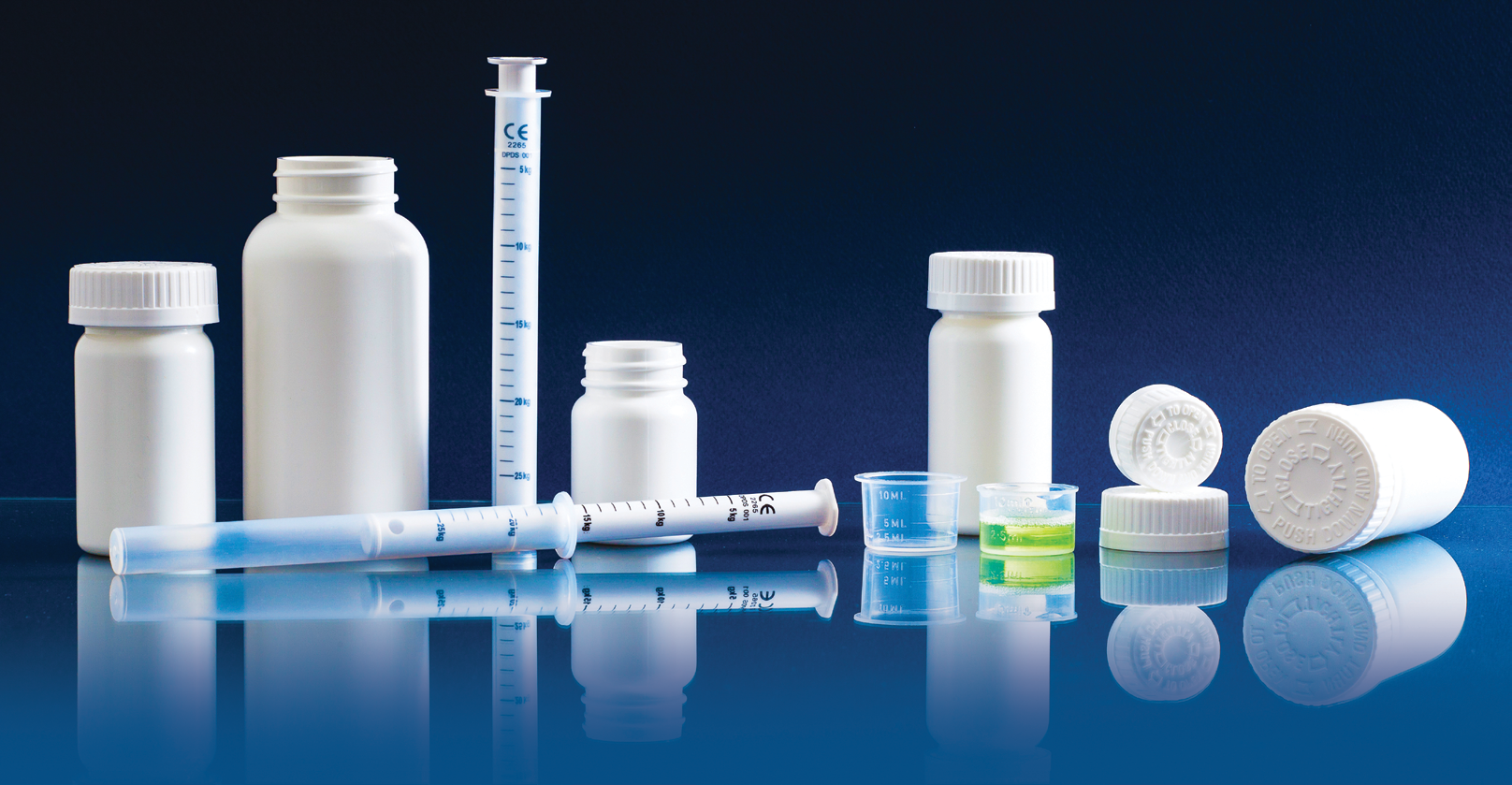
We specialize in the design, development, and manufacturing of primary packaging, drug delivery systems, medical devices, and customized plastics components. Besides our proprietary product, customized product development and contract manufacturing services are offered. Special emphasis is given to enhanced patient-friendly and child safety. Besides other benefits, our innovations focus on making the products affordable in healthcare dispensing systems, medical devices, primary packaging, drug delivery systems, diagnostic drop control dispensers, Needle safe systems, vials, container closure systems, oral dosage syringes, measuring cups, etc. for over the counter and prescription drugs.

Benefits of working with Doctor Pack
In-house Innovative Design & Development Team: Our Development Process starts with an understanding of your specific requirements. Our dedicated team of product designers and technical experts work on giving you innovative options with a scientific approach. 3D modeling, 3D printing, and SLA model submission of the development product eliminates costly mistakes.
In-house Mould Making setup: Product development is further facilitated and expedited by our Micro Molding capabilities. Our in-house mould-making facility is equipped to work on projects with a high degree of complexity and reduce time to develop and market for our customers.
Product Testing and Validation: The product passes through a comprehensive testing and validation process during the development stage; this assures the product’s performance throughout the product life cycle.
High-level documentation: We are well accustomed to strict demands on quality, timing, and production, giving Doctor Pack a competitive edge. At Doctor Pack, we have good exposure to the preparation and submission of apt documents for DHF, DMR, Risk Management ‘CE’ marking, ‘Type-III DMF’, 21 CFR 820, and the 510 (k) requirements.
One-stop-solutions
Doctor Pack Provides one-stop solutions for Product Design and development, Mould Development, Raw material Selection, Mould Development & Validation, Product Testing and validation, Regulatory Documentation support, and Clean room manufacturing. By integrating an additional piece of the supply chain Doctor Pack can offer the full one-stop-shop contact, from product design to distribution. For customers, this translates to:
- Faster time to develop and Market
- Improved product Quality
Decreased project time
- One fixed point of responsibility


About Our Company
Doctor Pack is a trusted Packaging and Device partner to leading global healthcare, pharmaceutical, and diagnostics companies, hospitals, retail pharmacies, and contract packagers.
We specialize in the design, development and manufacture of primary packaging, drug delivery systems, medical devices, and customized plastics components. Special emphasis is given to enhanced patient-friendly and child safety. Besides other benefits, our innovations focus on making the products affordable in healthcare dispensing systems, Medical Devices, Primary packaging, diagnostic devices such as Lancing Devices, Need safe systems, vials, and closures for over-the-counter and prescription drugs.



