- CGMP Complied, Validated Clean Room facility with latest equipment
- Products comply with USP, EP, BP,JP & other regulatory standards
- Access to Large Device Design Database
- High level documentation support for ‘CE’ Marking, Canadian & US DMF, US FDA 510(k), 21CFR 820
- IPR Protection Guaranteed – many patents are filed
- Dedicated Innovative Product Development team
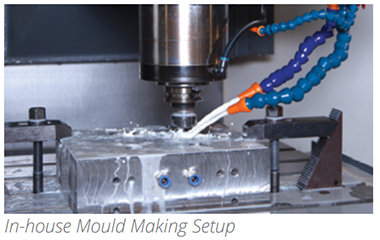
- In-house Molding Making and Micro Moulding facility for quicker Development and sample generation